NMR Part 1 Solutions: #5
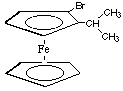
5.* (1996 2 6) A.How many different 13C NMR signals do you expect from the following ferrocene derivative (ignore spin coupling)?
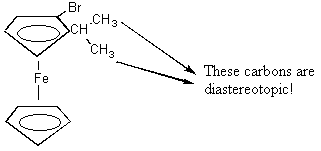
9. Since the rings rotate quickly with respect to one another, all of the signals from the carbons on the bottom ring are the same--they will overlap. This is the first signal. On the top ring there are 8 different C's and you guessed it--they all give different signals. The 5 ring C's give different signals because they're all at different positions with respect to the bromine and the alkyl substituent. But then why do the two methlys have different signals? They are diastereotopic (that was a hidden trick in this problem) because they're attached to carbon that is attached to something chiral (the rest of the molecule is non-superimposable on its mirror image). B. On the structure below write arrows with numbers, etc. to each different carbon. Remember that the rings rotate rapidly with respect to one another. Warning! This question contains a hidden trick.
|