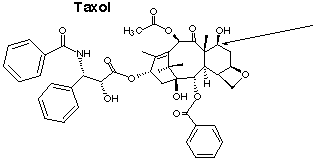
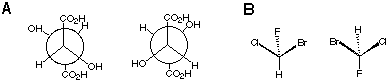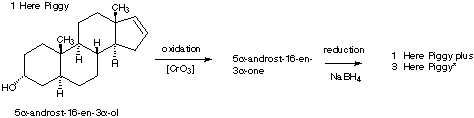1.* (1997 F 6) Taxol is an exciting anticancer agent for the treatment of advanced ovarian and breast cancer. Taxol is derived from the bark of the yew tree (which kills the tree) and by chemical modification of more readily available natural product from the needles of the yew. The structure of taxol is shown below.
A. Place an asterisk beside each chiral carbon in taxol. B. What is the absolute configuration of the rightmost carbon attached to an OH (labelled with an arrow in the figure). C. How many sites of unsaturation are in taxol?
2.* (1997 F 7) Which of the following pairs represent: (1) enantiomers, (2) diastereomers, (3) constitutional isomers, (4) identical subtances? Which are (5) optically active, (6) optically inactive? A. Write the appropriate number next to the letter for each substance. B. What is the name of the molecule shown on the left side of the last pair?
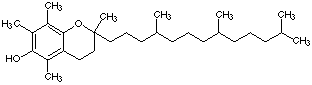
3.* (1996 F 4) Vitamin E is a fat soluble vitamin essential for muscle development; its structure is shown below. Two chemistry 32 students take Vitamin E; Fred gets his vitamin from a discount drug store; Sara believes in "natural foods" and so she buys her Vitamin E from a health food store. Fred’s Vitamin E is made in a factory; Sara’s Vitamin E is derived from soybeans. Fred and Sara compared their Vitamin E samples by taking a melting point and measuring the 1H NMR spectrum of each. The melting points are different!!
Vitamin E A. What is the difference between Fred and Sara's samples? B. What additional experiment would clarify the difference between Fred and Sara's Vitamin E? C. Would the NMR spectra of the two samples be the same? D. What do you advise Fred and Sara about taking the synthetic versus natural Vitamin E?
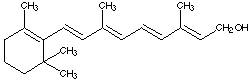
4.* (1996 F 5) Vitamin A or retinol is an essential component for the vision receptors in the eyes. Its structure is shown below. Reaction of Vitamin A with H2 over Pd/C produces a substance whose molecular formula is C20H40O. This substance appeared impure; for example it showed a wide melting range and several bands on a chromatographic column. Explain the nature of this new material and comment on its optical activity.
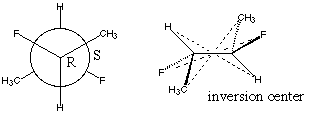
5.* (1996 F 6) The complete Newman formula for 2S, 3R difluorobutane is shown below, along with another diagram.
What is the relationship between this compound and 2R, 3S difluorobutane? C. Comment on the optical activity of these compounds.
6.* (1996 1 3) Below is shown the structure of a naturally-occurring male sex pheromone found in pigs: 5a-androst-16-en-3 a-ol. This compound, called "Here Piggy," is said to have a musk odor, and roughly one-half of the humn population can detect this odor. Of those, about half find the odor attractive; the other half find it repulsive. Male pigs (boars) made and excrete Here Piggy from their saliva; this odor sexually arouses female pigs (sows). The same compound (Here Piggy) has been isolated from the delectable fungus, truffles. In France, pigs are used to detect truffles under the ground.
A. Write down the molecular formula for Here Piggy. B. How many sites of unsaturation are there in Here Piggy? Identify them in a short statement. C. Place an asterisk beside each chiral carbon on the structure shown for pheromone Here Piggy. How many stereoisomers could exist for this general structure? Show your calculations. D. The pheromone Here Piggy can be oxidized into another compound, 5 a-androst-16-en-3-one, which has two fewer hydrogen atoms. Write a structural formula for this new substance by making use of your knowledge of nomenclature. When 5 >b>a -androst-16-en-3-one was treated with a reducing agent selective for carbonyl derivatives, two isomeric products were formed; one is identical to the original Here Piggy.E. Draw the a structural formula for the other isomer of Here Piggy showing the stereochemistry. F. What term describes the relationship between the original Here Piggy and its isomer? G. Constrast the odor and optical activity you might expect to be manifest by these two isomers.
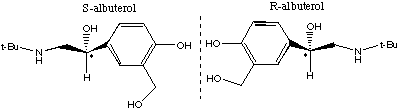
7.* (1995 F 4) According to the Wall Street Journal (Nov 7, 1995) "many drugs have the personalities of Dr. Jekyll and Mr. Hyde: they relieve pain but also upset the stomach." By separating the "good molecules" from the "evil" twins, researchers can reduce the negative side effects of some drugs. A case in point is S-albuterol (the bad twin) and R-albuterol (the good twin). The former increases the severity of asthma attacks whereas the latter relaxes bronchial muscles. Structures of S- and R- albuterol are shown below in chicken-wire notation.
A. Explain the different physiological effects of such twins. B. Discuss some physical properties of the twins. Which would be the same and which would be different? C. How did these twins get their names "S" and "R"?
8.* (1995 1 1E) Show a Newman projection along the C2-C3 bond of 2R, 3S dichlorobutane. Is the compound optically active?
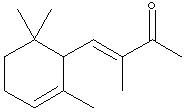
9.* (1995 1 2) The perfume industry can be traced back through many civilizations; for example, in the 5th centruy BC Herodotus included a chapter on perfumes in his "Histories." In modern times, some perfumes are made by synthetic organic chemists, while others are isolated from natural products. Consider a-isomethylionone, X, a component derived from violet flower oils. The structural formula of X is shown below in chickenwire notation.
A sample X (Xn) derived from natural flower oils is optically active; however, a sample of X (Xs) produced from a total synthesis does not rotate plane-polarized light even though this synthetic sample, Xs, exhibits both an infrared spectrum and 1H and 13C-NMR spectra identical to those of the sample Xn derived from natural sources. The natural and synthetic samples Xn and Xs have different fragrances. Catalytic hydrogenation of one mole of either Xn or Xs over a Pd catalyst took up to 2 moles of H2 (as indicated below). The product Ys from Xs was shown to be a mixture of 4 components. When the 4 components of product Ys were separated with a gas chromatograph, each component showed the same molecular formula (C14H26O) as determined by mass spectrometry. Xn, Xs, and the 4 components of Ys show a strong infrared peak in the region associated with a ketone (1690-1700 cm-1). On the basis of this information answer the following: A. How many sites of unsaturation are in X and Y, respectively? B. Write an asterisk beside each chiral center in the structural formula shown for X.
C. Identify the nature of the apparent stereoisomers: Xn and Xs. D. Write the structural formula for Y in chickenwire notation. E. Place asterisks beside each chiral center on your structure for Y. F. What is the nature of the four stereoisomeric components represented by Y? A simple term will do. G. Using the terms R and S, explain why Yn would exist as 4 stereoisomers.
|