|
SME Light Winter Quarter Lecture 10: Where Do Molecules Fit Into This Picture? Thursday, February 3rd, 2000 Outline
I. Organic notation Materials needed:
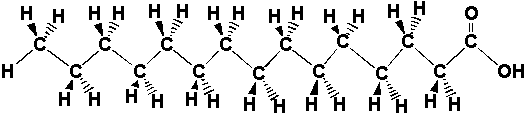
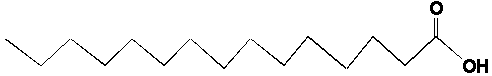
I. Organic notation Unfortunately, it's difficult to do things interactively or in 3D all the time. Therefore, chemists have developed a two-dimensional shorthand to depict molecules. Following we will see some features of this shorthand. A. Leaving out the hydrogens and the carbons Often things just start looking to busy if we write in the letter for every atom. One way that chemists get around this is that they don't show letters for the carbon, and they leave out even the bonds to hydrogen. It is possible to do this because it is a rule that carbon forms four bonds and that hydrogen only forms one bond. If ever a carbon looks like it has only two bonds, that is just because two of its bonds (to hydrogens) are not shown. Below are some representations of a fat molecule in longhand and shorthand. We will talk more about fat molecules later in the lecture. See how much simpler it is to represent carbon chains without having to write in all those bonds and letters?
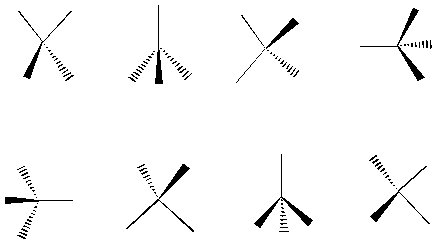
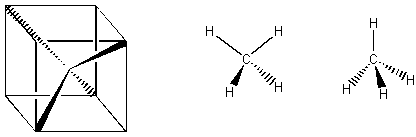
 B. Bold and dashed lines When you see a bold line, that represents something coming out of the plane of the paper. When you see a dashed line, that represents something going into the plane of the paper. When you see a normal line, it is in the plane of the paper. Using this notation, can you see how the following structures are all different representations of tetrahedra? By the way, can you tell that each bond of the tetrahedron is an three-fold axis of rotation for the figure?
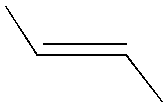
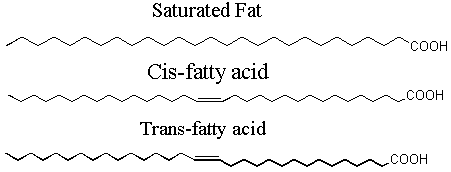
 II. Stereoisomers Stereoisomers are molecules in which every atom is hooked up to the same other atoms, but that are in some way different. How are they different? Symmetry!! First, one important caveat: we are not talking here about molecules that differ by rotation about a single bond. (Single bonds are when you see just one bond-line between the two atoms.) The two molecules below are not stereo-isomers; they are the same molecule. Every single bond exhibits free rotation. Double bonds (in which there are two bond-lines between the atoms) cannot rotate freely. The reason for this is deep and interesting, but we won't go into it here. A. Double bond stereoisomers: the story of margarine Molecules of fat are mostly made up of long chains of carbon atoms with a COOH on the end. Sometimes, a fat molecule will have a double bond in the middle. When a fat molecule has a double bond, it can't have as many hydrogens on the chain as it had without the double bond. When there are as many hydrogens as there can possibly be (i.e. no double bonds), the molecule is "saturated" with hydrogens. For this reason, fat molecules that have no double bonds are called saturated fats. At room temperature, saturated fats are generally solid, like butter. When a fat molecule has a double bond, there are some interesting consequences. Unlike for single bonds, there is no free rotation about double bonds. If something is on one side of the double bond, it has to stay on that side. Second, putting in a double bond means that there is less room for hydrogen in the molecule. For this reason, a fat molecule possessing one or more double bonds is called an unsaturated fat. In nature, unsaturated fats always have the carbon chains on the same side of the double bond. They are called cis-fatty acids. At room temperature, unsaturated fats are typically liquid oils. When butter supplies were low just after WWII, people wanted a non-liquid fat alternative, and the industry responded by chemically removing some of the cis (carbon chains on the same side) double bonds in oils and replacing them with trans (carbon chains on the opposite side) double bonds. What resulted was a transparent, semi-solid block of "margarine" that spread more easily than butter and proved very popular (after being artificially colored yellow).
 B. Carbon's Tetrahedral Geometry: "The Magic of the Tetrahedron" Carbon bonds to four different things, and the electrons in its bonds naturally repel each other. Take one color of gumdrop and four of a second color of gumdrop. With toothpicks, make four bonds coming out of the carbon in the middle. Remember that electrons repel each other, so keep the toothpicks as far apart as you can!! If the toothpicks are really as far apart as they can possibly be, then you will have made a tetrahedron. Did you? If not, can you see how you can get your bonds even farther apart via using tetrahedral geometry?? If carbon is attached to four atoms that are the same, like four hydrogens or four chlorines or something, then the geometry of the resulting molecule is tetrahedral. Can you find all of the symmetry elements in the molecule of methane (CH4) below? Do you see that each carbon-hydrogen bond is a three-fold rotation axis?
 Now look at the two molecules below. Build a model to help you see them better.
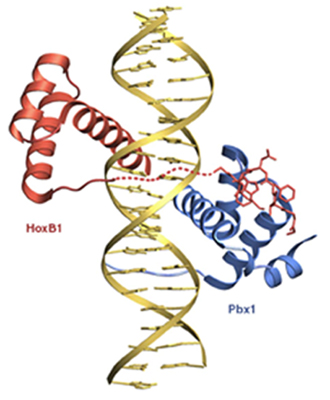
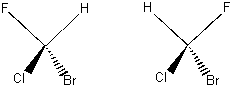 When the tetrahedron is "decorated" with hetero-atoms, its symmetry is destroyed. Chiral molecules are different in some geometric way, even though each atom is attached to the same other atoms. You can't make these molecules into the same thing, no matter how hard you try and no matter how much it looks like you should be able to. They are called enantiomers. This abstract and seemingly small difference in symmetry has vast implications in our bodies and in the world. Before people knew that enantiomers do different things in your body, they noticed that they do different things with light. In particular, plane polarized light. If you take plane polarized light and shine it through a solution of one pure enantiomer, the plane of polarization of the light that comes out the other side will be rotated a little bit!! And then if you take the other pure enantiomer and shine plane polarized light through it, the plane of polarization of the light that comes out the other side will be rotated a little bit in the opposite direction!! The reason is that, in a nutshell, the two enantiomers have opposite handedness, so they interact differently with the handed circular components of the plane polarized light. You will get a chance to explore this more deeply in lab this week. C. Where does it all come from? Most biological molecules come as one enantiomer. The other enantiomer simply isn't recognized by your body. We would say that these molecules are handed, or chiral. All of your amino acids (the biological building blocks of proteins) are chiral. Amino acids that have the opposite handedness won't work: your body simply won't recognize them. What's more, the amino acids are all left-handed (more on how we define right handed and left handed for molecules in a later lecture). By contrast, biologically useful sugars such as dextrose (and corn syrup, which you'll see in lab this week) are all right-handed. Interestingly, your amino acids all rotate ultraviolet light in the same direction (only ultraviolet, though: the visible and infrared wavelengths are rotated in unpredictable directions). The formal definition of chirality is that a chiral molecule has a non-superimposable mirror image. If you take models of the two molecules above and try to superimpose them, you will find that you can't. The mathematical way to tell whether an object is chiral is to see whether it has a symmetry operation that is an n-fold rotation followed by reflection through a mirror plane perpendicular to the n-fold rotation axis. If it's easier for you to look for non-superimposable mirror images, though, just do that. DNA is chiral-in nature, it always spirals the same way, or has the same handedness. Below is a picture of DNA: look at all the spirals!
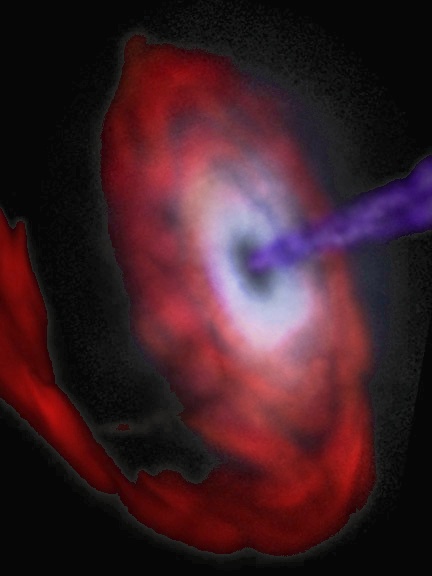
 Interesting molecular level stuff, eh? But this stuff isn't limited to the molecular level! Currently astronomers are studying the death spirals of black holes and/or neutron stars. When the new LIGO probe at Caltech starts taking data sometime around 2002, it should be able to detect the final "chirp" of gravitational radiation produced by a pair of inspiralling neutron stars in the Virgo Cluster, a cluster of galaxies almost 50 million light years away.
 Some references for this stuff:
Okay, why?!! After all, there's no fundamental difference between left or right, is there? Actually, the weak nuclear force (one of the four fundamental forces in physics) distinguishes between the two! When radioactive atoms decay via the weak force, the electrons they shoot off tend to have a left-handed spin. Could this affect the handedness of molecules or crystals that happen to be forming in the vicinity? Some experiments suggest this might be true. Some physicists theorize that chirality came from outer space, where a rotating neutron star with an intense, circularly polarized gravitational field emitted ultraviolet light. The handedness of this light could then influence the molecules being formed or degraded in the thin interstellar gas. If these molecules made their way to earth at the appropriate time, their enzymatic amplification could be responsible for the handedness of molecules in our bodies today. In 1997, scientists extracted amino acids from a meteorite and found a slight prevalence of one handedness over the other. People are skeptical of whether this proves the "chirality comes from space" hypothesis, but it certainly is interesting, isn't it?
|